Systems biology can describe intricate responses to therapeutic interventions in pre-clinical studies and also provide predictive models for drug safety and efficacy before going for the clinical phases. Systems biology can potentially reduce the R&D expenditure and time by improving target identification and lead quality, better description of pharmacokinetic and toxicity profiles, as well as optimizing clinical trial efficiency.
Before embarking on the costly clinical phases of drug development all the tools predicting the probability of success are always a welcome and so is systems biology. There is increasing realization that regarding the need for CROs to diversify. Early phase contract research organizations (CROs) can develop their capacity to complement the pharmaceutical or biotech industry by making use of in-silico disease models and a virtual patient approach for the identification and validation of targets, develop biomarkers to improve the success rates of clinical trials, and thus expedite the therapeutic translation.
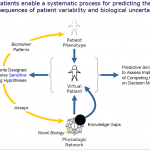
By integrating the data (genomic, proteomic, physiologic, environmental) for modeling the disease systems and further creation of virtual patients representing real patient population enables running clinical trials in silico. This biosimulation approach allows for clinical protocol decision making and helps in deciding the optimal design of clinical trials. It is cost effective, saves time, and reduces the number of patients required for clinical trials. It facilitates the go-no-go decisions and may also help in in-licensing opportunities at this stage. Virtual patients allow different combinations of biological attributes to be evaluated in order to identify biomarkers. [2]
Virtual patients are explicit, quantitative representations of known or hypothesized biology underlying both normal physiology and disease pathophysiology. Virtual patients can represent healthy phenotypes, different phenotypes with similar underlying pathophysiology, the same phenotype with different pathophysiologies, or patients at different stages of disease progression. [1]
Fig. 1. Virtual Patients [2]
This concept of computer based predictive biosimulation by top down mechanistic modeling extends to BA/BE studies as virtual bioequivalence trials and can provide an understanding of the complex interactions that exist among physicochemical properties, physiological variables, pharmacokinetics and formulation variables, and with that understanding, contribute to reducing the risk of failure.
Predictive simulations of anatomical, physiological and pathological data is being integrated as a Virtual Physiological Human (VPH) that, once established, will enable collaborative investigation of the human body as a single complex system. [3]
References
[1] http://www.entelos.com/pubArchive/PAREXEL_FINAL.pdf [2] http://www.fosbe.org/slides/Trimmer.pdf
Woltosz WS, Chittenden J, DiBella J, Virtual Bioequivalence Trials Can Help Reduce Risk of Failure, Simulations Plus, Inc., FDA Science; http://www.accessdata.fda.gov/scripts/oc/scienceforum/sf2005/search/preview.cfm?abstract_id=528&backto=author
[3] Virtual Physiological Human Network of excellence, http://www.vph-noe.eu/objectives/vph-for-the-public